Fluorescent Proteins
What Are Fluorescent Proteins?
Fluorescent protein is a broad term with a few different meanings in biology. In general, it refers to proteins that naturally emit light (fluoresce) when exposed to certain wavelengths of light without the need for dyes or enzymes. Fluorescent proteins are some of the most common tools in modern molecular biology, and they enable a wide variety of diverse research applications.
Biologists around the world employ hundreds of variants of fluorescent protein in their research to study various aspects of living systems in real time. In fact, there are now engineered or “enhanced” varieties for almost every color of the rainbow (and beyond). Two of the most common types of fluorescent protein are green fluorescent protein (GFP) and red fluorescent protein (RFP). While most fluorescent protein variants are primarily used in microscopy, GFP and RFP are much more common in macroscopic imaging.
The Odyssey M Imager and Odyssey F Imagers both support dozens of varieties of fluorescent protein imaging, and the Odyssey XF Imager supports yellow fluorescent protein (YFP), RFP, and iRFP imaging. Refer to the compatibility table below to find the right Odyssey Imager for common variants of fluorescent protein.


1. Green Fluorescent Protein (GFP)
Green fluorescent protein was the first type of fluorescent protein to be discovered and widely used in biology. Originally isolated in 1961 and cloned in 1992, the wild-type peptide paved the way for countless colorful mutations and fusion proteins.[1, 2] In a sense, the development of all other fluorescent protein variants, probes, and fusion products can be attributed to the first uses of GFP.
GFP naturally fluoresces green when exposed to blue or ultraviolet light. Today it is the industry standard for fluorescent protein, and it is often used as a reporter of gene or protein expression. Though GFP is a small protein containing only 238 amino acids with a weight of 27 kDa, its entire molecular structure is critical to its fluorescent properties.
It's worth noting that the term “green fluorescent protein” may refer to one specific protein or a group of several proteins with similar properties. For instance, enhanced green fluorescent protein (EGFP), Emerald, and mWasabi are all engineered variants of the original wild-type GFP. However, these may all collectively be called “green fluorescent proteins” because they share similar excitation and emission profiles. This also holds true for other varieties like red fluorescent proteins.

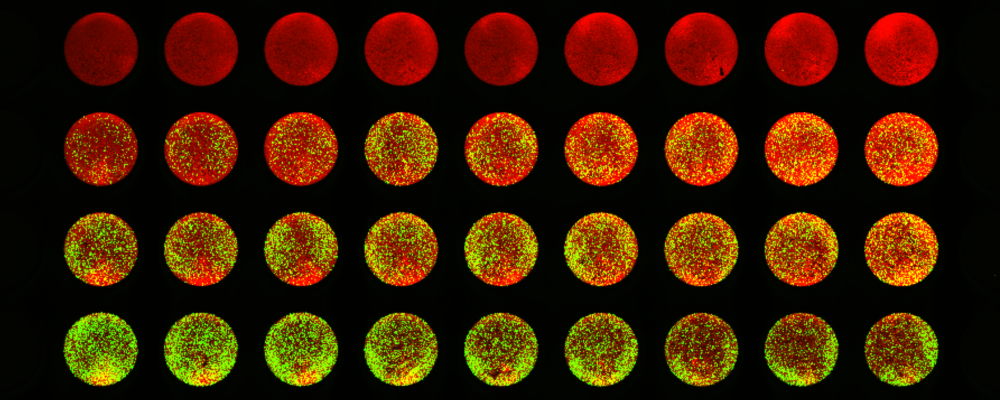
2. Red Fluorescent Protein (RFP)
Red fluorescent protein is another common type of fluorescent protein. RFP was first isolated from coral and then developed into enhanced variants such as dTomato and mCherry.[2] It is often paired with certain GFPs to enable multiplex fluorescence imaging of cells.
RFP naturally fluoresces red-orange when exposed to green or yellow light (depending on the variety), and it features a molecular structure similar to GFP. However, RFPs are generally more suitable for optical imaging and in vivo applications than GFPs due to reduced autofluorescence, light scattering, and phototoxicity at longer wavelengths.[3]
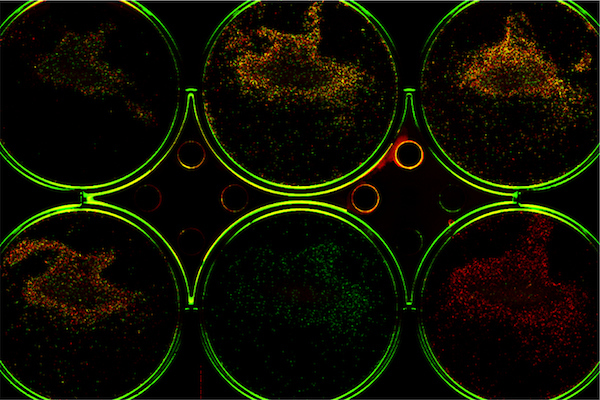
3. Infrared Fluorescent Protein (iRFP)
In 2009, a new class of fluorescent proteins was established with the first infrared fluorescent protein (iRFP).[4] This protein class is particularly well suited for in vivo imaging, as their wavelengths penetrate tissue well and benefit from greatly reduced autofluorescence.[5, 6, 7] Unfortunately, some microscopes and plate readers are not adequately equipped for near-infrared (NIR) fluorescence imaging greater than 650 nm.
The Odyssey M Imager and Odyssey F Imagers are both designed to enable NIR multiplex imaging, allowing the use of this class of fluorescent proteins for cell-based assays while taking advantage of the significantly lower background autofluorescence in this region. The Pearl® Trilogy Imager also supports the use of iRFP for in vivo optical imaging.
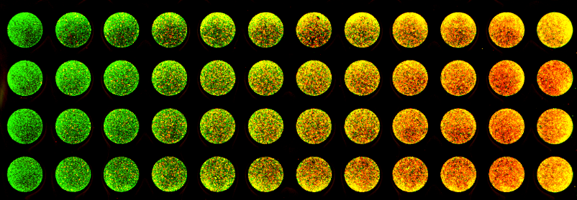
Common Types of Fluorescent Protein Assays
Biologists use fluorescent proteins to perform an ever-expanding variety of cutting-edge applications. They are used to study signaling pathways, protein expression, cell lineage tracing, protein-protein interactions, and organelle structure and activity, just to name a few. Here we discuss two of the most common uses of fluorescence proteins, which are monitoring protein expression and cell and tissue labeling.
1. Expression Assays
Most protein expression assays fall into one of two categories: protein expression levels and protein localization. For these assays, an expression vector is often created that contains the coding sequence for a protein of interest and a fluorescent protein. This creates what is known as a chimeric fusion protein. Then this vector is transfected into a cell line that can express the fusion protein. Finally, cells are observed using either a plate reader or a fluorescence microscope to determine expression levels or where the protein is expressed within the cell. Protein localization assays are qualitative rather than quantitative expression assays.
If your goal is to determine expression levels, then this assay may be performed on a plate reader. However, plate readers only provide quantification values; you don’t get to visualize the plate’s wells or the proteins themselves. It is just a number without context. This is where our Odyssey Imaging Systems offer a major advantage over traditional plate readers. They provide the actual plate image as well as the quantification that correlates with each well. This allows you to both see and quantify your protein expression assay. Plus, by multiplexing channels on your Odyssey Imager, you can visually verify if cell number or protein expression has truly changed. This delivers a much more complete story than what any traditional plate reader alone can provide.
Our Odyssey Imaging Systems deliver the best of both worlds for studying and quantifying protein expression. They provide both protein expression values as well as brilliant digital fluorescence imagery often only found in microscopy. Our instruments show and tell the entire protein expression story unlike a traditional plate reader or microscope alone.
2. Cell and Tissue Labeling
Fluorescent proteins can also be used to label subcellular structures, whole cells, and tissues. These types of assays are often performed on slides containing live cells, whole organisms, or excised tissues, and they can provide valuable insight into the dynamics of biological systems. Researchers employ multiplex labeling techniques to visualize specific cell types within whole organisms, organs, tissues, and cell culture, monitor cell cycle progression, identify organelles and subcellular structures, and study cell and enzyme behavior in certain environments.
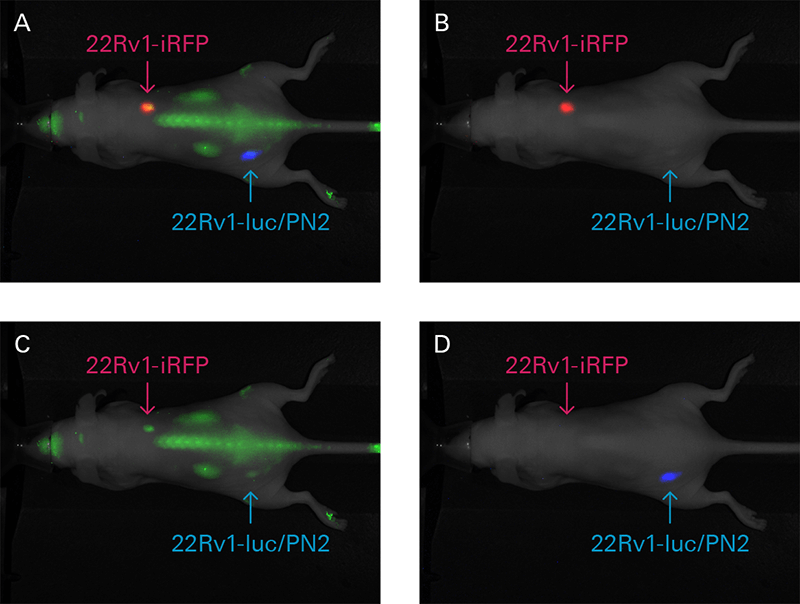
A limitation of fluorescent imaging within whole animals is light absorption by melanin and hemoglobin, as well as light scattering. As the light wavelength increases, the impact of both decreases, with an ideal range between 650 – 1,100 nm. Through NIR fluorescence imaging, scientists can achieve deeper excitation light penetration, exceptional sensitivity, and reduced autofluorescence in both live animals and excised tissues. For example, the Pearl Trilogy Imager can be used to monitor cancer cells and tumor growth or track therapeutic response in mice with iRFP.
Considerations for Fluorescent Protein Assays
There are several factors to consider when it comes to designing fluorescent protein assays. Some key concepts include excitation and emission profiles, stability, brightness, phototoxicity, and in vivo structure. Luckily, you don’t have to tackle these challenges alone. Your dedicated Solutions and Support Scientist will gladly work with you to optimize your lab’s fluorescent protein assays. Here are a few of the most important considerations to keep in mind:
- The half-life of your fluorescent protein variant(s)
- The brightness of your fluorescent protein variant(s)
- The stability and degradation of your fluorescent protein fusion
- The potential cell toxicity of your fluorescent protein expression
- The emission and excitation profiles of your fluorescent protein variant(s)
- How your instrument aligns with your fluorescent proteins’ emission and excitation profiles
- Your instrument’s channels and which are ideal for quantification
- Is the goal of your assay expression (quantitative) or merely visualization (qualitative)?
- Intrinsic protein properties may be affected by tagging with fluorescent protein
- Which variant is ideal for your target, sample, instrument, and experimental design?
- The experimental cost, as some expression vectors are much more expensive than others
- The availability of your instrumentation and fluorescent protein variant(s)
- Will your fusion protein itself affect protein expression or localization?
References
- Shimomura, O., Johnson, F.H. and Saiga, Y. (1962), Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J. Cell. Comp. Physiol., 59: 223-239. https://doi.org/10.1002/jcp.1030590302
- Introduction to fluorescent proteins. Nikon’s MicroscopyU. (n.d.).
- Shcherbakova DM, Subach OM, Verkhusha VV. Red fluorescent proteins: advanced imaging applications and future design. Angew Chem Int Ed Engl. 2012;51(43):10724-10738. doi:10.1002/anie.201200408
- Doerr, A. Fluorescent proteins: into the infrared. Nat Methods 6, 482–483 (2009). https://doi.org/10.1038/nmeth0709-482a
- Hock, A.K., Cheung, E., Humpton, T.J. et al. Development of an inducible mouse model of iRFP713 to track recombinase activity and tumour development in vivo. Sci Rep 7, 1837 (2017). https://doi.org/10.1038/s41598-017-01741-0
- Hock, A. K., Lee, P., Maddocks, O. D., Mason, S. M., Blyth, K., & Vousden, K. H. (2013). iRFP is a sensitive marker for cell number and tumor growth in high-throughput systems. Cell Cycle, 13(2), 220–226. https://doi.org/10.4161/cc.26985
- Goto, M., Ryoo, I., Naffouje, S., Mander, S., Christov, K., Wang, J., et al. Image-guided surgery with a new tumour-targeting probe improves the identification of positive margins. eBioMedicine, Volume 76, 2022, 103850, ISSN 2352-3964, doi.org/10.1016/j.ebiom.2022.103850.
- Henry, M.D., et al. (2005), Spiculated periosteal response induced by intraosseous injection of 22Rv1 prostate cancer cells resembles subset of bone metastases in prostate cancer patients. Prostate, 65: 347-354. https://doi.org/10.1002/pros.20300
