Recent blog posts have highlighted some of the most exciting clinical developments of IRDye® near-infrared fluorescent dyes as surgical aides. Beyond these examples, IRDye infrared dye products are being used in more than 20 clinical trials around the globe, many of which involve the deadliest and most common cancers.
Brain and Pancreatic Cancer
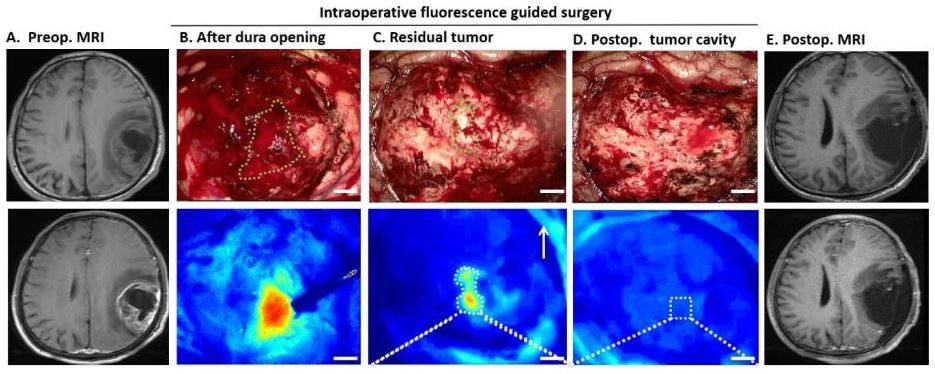
Glioblastoma and glioma brain and pancreatic adenocarcinoma tumors are particularly aggressive forms of cancer that are difficult to treat. IRDye dye-conjugated optical probes are currently being investigated as an alternative to traditional surgical treatments for these cancers. Very recently in April 2018, Deling Li and colleagues announced the results their first-in-human study a novel 68Ga-IRDye 800CW-BBN positron emission tomography (PET) and near-infrared fluorescent (NIRF) dual modality optical probe in patients with glioblastoma multiforme (GBM) [1, 2]. The authors used preoperative PET and intraoperative fluorescence-guided surgery methods, demonstrating that the “novel dual model imaging technique is feasible for integrated pre- and intra-operative targeted imaging via the same molecular receptor improved intraoperative GBM visualization and maximum safe resection” [1]. GBM is the deadliest and most aggressive glioma type, and novel GBM therapies have the potential to impact many lives.
Breast and Colorectal Cancer
Breast and colon cancers are some of the most common cancers around the globe with millions of cases diagnosed each year. Two very recent clinical trials by G.M. van Dam at University Medical Center Groningen in collaboration with Martini Hospital Groningen and UMC Utrecht have evaluated the anti-vascular endothelial growth factor antibody-IRDye 800CW-bevacizumab conjugate in breast cancer study [8, 9]. van Dam’s studies are currently assessing dosing, uptake, quantification, and localization of the optical probe, as well as determining if the conjugate is appropriate for intraoperative breast cancer surgery [8, 9].
Learn More About Optical Probe Validation and Parameters.
Colorectal cancer is also a very common diagnosis around the world. Two recent clinical trials by W.B. Nagengast of the University Medical Center Groningen evaluated IRDye 800CW-bevacizumab for colorectal cancer diagnosis [10, 11]. Nagengast noted “there is a need for better visualization of polyps during surveillance endoscopy in patients with hereditary colon cancer syndromes like Familial Adenomatous Polyposis (FAP) and Lynch Syndrome (LS), to improve adenoma detection rate,” also stating that “optical molecular imaging of adenoma associated biomarkers is a promising technique to accommodate this need” [10]. In addition to detection, IRDye 800CW-bevacizumab is also being investigated as an aid for narrowing down specific colon cancer management surgeries and therapies [11].
Other Clinical Applications
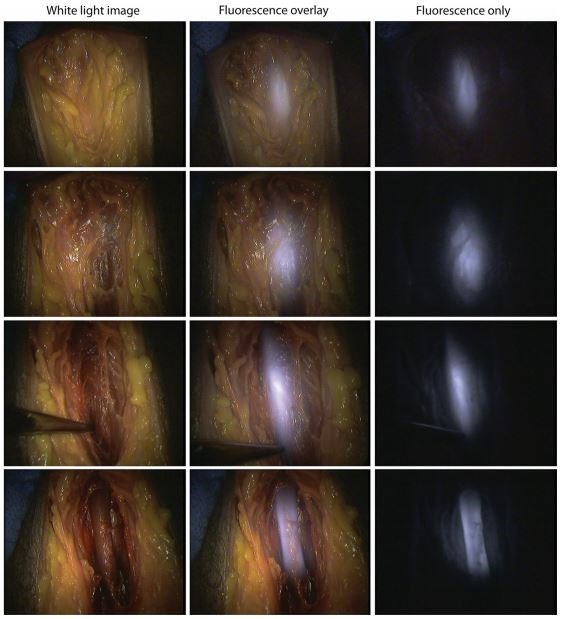
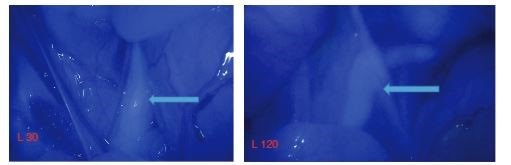
While the focus of this blog post series has been on pre-clinical and clinical applications of IRDye conjugates for cancer, these are not the only applications. Currently, IRDye fluorophores are being evaluated in several trials for clinical use in non-cancer surgeries, like abdominal and urological. Ureters, the path for urine between the kidneys and bladder, and the urethra, often present difficulties in abdominal and urological surgery. By illuminating these pathways with fluorescent dyes, the anatomy is bright and clear, which may allow surgeons to more precisely navigate around them during surgery. A study published in 2017 by T.G. Barnes et.al. in Techniques in Coloproctologydemonstrated urethra illumination in cadavers with IRDye 800BK as a method for enhancing low rectal surgical navigation [15]. The authors concluding that “IRDye 800BK is a promising alternative to ICG [indocyanine green] in visualizing the urethra,” and that “Its greater depth of penetration may allow earlier detection of the urethra during surgery and prevent wrong plane surgery sooner” [15].
Conclusion
This blog series on optical surgery navigation has illuminated the potential of IRDye fluorescent dyes as surgical aids. From the deadliest cancers to routine, minimally-invasive gynecological surgeries, IRDye fluorophores present a valuable opportunity for visualizing, understanding, and ultimately treating various diseases. More information about the studies mentioned can be found at ClinicalTrials.gov at the locations mentioned below.
IRDye fluorophores are only used for investigative purposes in clinical trials.
References
- Li, D., Zhang, J., Chi, C., Xiao, X., Wang, J., Lang, L., & Ali, I. (2018, April 3). First-In-Human Study of PET and Optical Dual-Modality Image-Guided Surgery in Glioblastoma Using 68Ga-IRDye800CW-BBN. Theranostics, 8(9), 2508-2520. doi:10.7150/thno.25599
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02901925, A Microdose Evaluation Study in Recurrent Glioma (ABY-029); 2016 December. Available from: https://clinicaltrials.gov/ct2/show/NCT02901925.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02910804, IRDye800CW-BBN PET-NIRF Imaging Guiding Surgery in Patients With Glioblastoma; 2015 November. Available from: https://clinicaltrials.gov/ct2/show/NCT02910804.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02855086, Cetuximab-IRDye 800CW in Detecting Tumors in Patients With Malignant Glioma Undergoing Surgery; 2016 October. Available from: https://clinicaltrials.gov/ct2/show/NCT02855086.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT03510208, Panitumumab-IRDye800 in Diagnosing Participants With Malignant Glioma Undergoing Surgery; 2018 May 14. Available from: https://clinicaltrials.gov/ct2/show/NCT03510208.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02736578, Cetuximab-IRDye800CW and Intraoperative Imaging in Finding Pancreatic Cancer in Patients Undergoing Surgery; 2016 July. Available from: https://clinicaltrials.gov/ct2/show/NCT02736578.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02743975, Near-Infrared Image Guided Surgery in Pancreatic Adenocarcinoma (PENGUIN); 2016 September. Available from: https://clinicaltrials.gov/ct2/show/NCT02743975.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02583568, Fluorescence Guided Surgery in Breast Cancer (MARGIN); 2015 October. Available from: https:/clinicaltrials.gov/ct2/show/NCT02583568.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT01508572, VEGF-Targeted Fluorescent Tracer Imaging in Breast Cancer; 2011 October. Available from: https://clinicaltrials.gov/ct2/show/NCT01508572.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02113202, Molecular Fluorescence Endoscopy in Patients With Familial Adenomatous Polyposis, Using Bevacizumab-IRDye800CW (FLUOFAP); 2014 March. Available from: https://clinicaltrials.gov/ct2/show/NCT02113202.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT01972373, Visualization of Rectal Cancer During Endoscopy, Using a Fluorescent Tracer (RAPIDO-TRACT);2013 October. Available from: https://clinicaltrials.gov/ct2/show/NCT01972373.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT03106038, Dose-Escalation Study of a Constrant Agent for Delineation of Urological Anatomy in Minimally Invasive Surgery; 2017 May 4. Available from: https://clinicaltrials.gov/ct2/show/NCT03106038.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT03387410, Ureter Identification with IRDye 800BK; 2018 April 6. Available from: https://clinicaltrials.gov/ct2/show/NCT03387410.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT02975219, Feasibility Study of Using Molecular Fluorescence Guided Surgery in Endometriosis (Endo-Light); 2017 May 1. Available from: https://clinicaltrials.gov/ct2/show/NCT02975219.
- Barnes, T. G., Volpi, D., Cunningham, C., Vonjovic, B., & Hompes, R. (2018, February 19). Improved Urethral Fluorescence During Low Rectal Surgery: A New Dye and a New Method. Techniques in Coloproctology, 22, 115-119. doi:10.1007/s1051-018-1757-6
- Al-Taher, M., van den Bos, J., Schols, R. M., Kubat, B., Bouvy, N. D., & Stassen, L. S. (2018, February 2). Evaluation of a Novel Dye for Near-Infrared Fluorescence Delineation of the Ureters During Laparoscopy. British Journal of Surgery BJS Open. doi:10.1002/bjs5.59
Powered by Froala Editor