Azide Group of Click Chemistry Reagents
Products
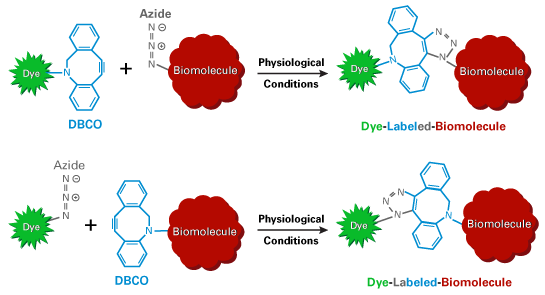
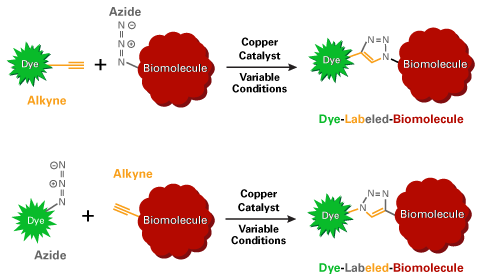
Azides preferentially label molecules containing either alkynes or DBCO groups. Copper-catalyzed click chemistry is used for initiating reactions between azides and alkynes, whereas azides spontaneously react with DBCO groups.
Within physiological temperature and pH ranges, the azide group does not react with amines or hydroxyls, which are naturally present in many biomolecules. However, the azide group is unstable towards sulfhydryls (–SH, thiols) and organophosphines such as tris(2-carboxylethyl)phosphine (TCEP).